Neural Organoid Platform™
Pharmaceutical development for human central nervous system (CNS) disorders currently use rodent model systems to approximate humans. Despite apparent success in rodent models, most drugs fail in subsequent human clinical trials precisely because they were neither developed in, nor evaluated against a fully human model. Hence, the neural organoid platform is both timely and highly relevant to the validated market opportunity to identify and evaluate potential drugs for toxicity, safety and efficacy on model systems that accurately mimic the human CNS.
Neurxstem is developing products initially delivered using a specialized contract research organization model. The company will provide fee-based testing for drug targets and candidate toxicity, safety and efficacy using its synthetic Neural Organoid Platform™.
Neural Organoid Platform™ addresses significantly all the needs of potential clients for disease mechanism research, target identification, and TSE needs of big Pharma in a “one stop” platform that is highly predictive of human neural physiology.
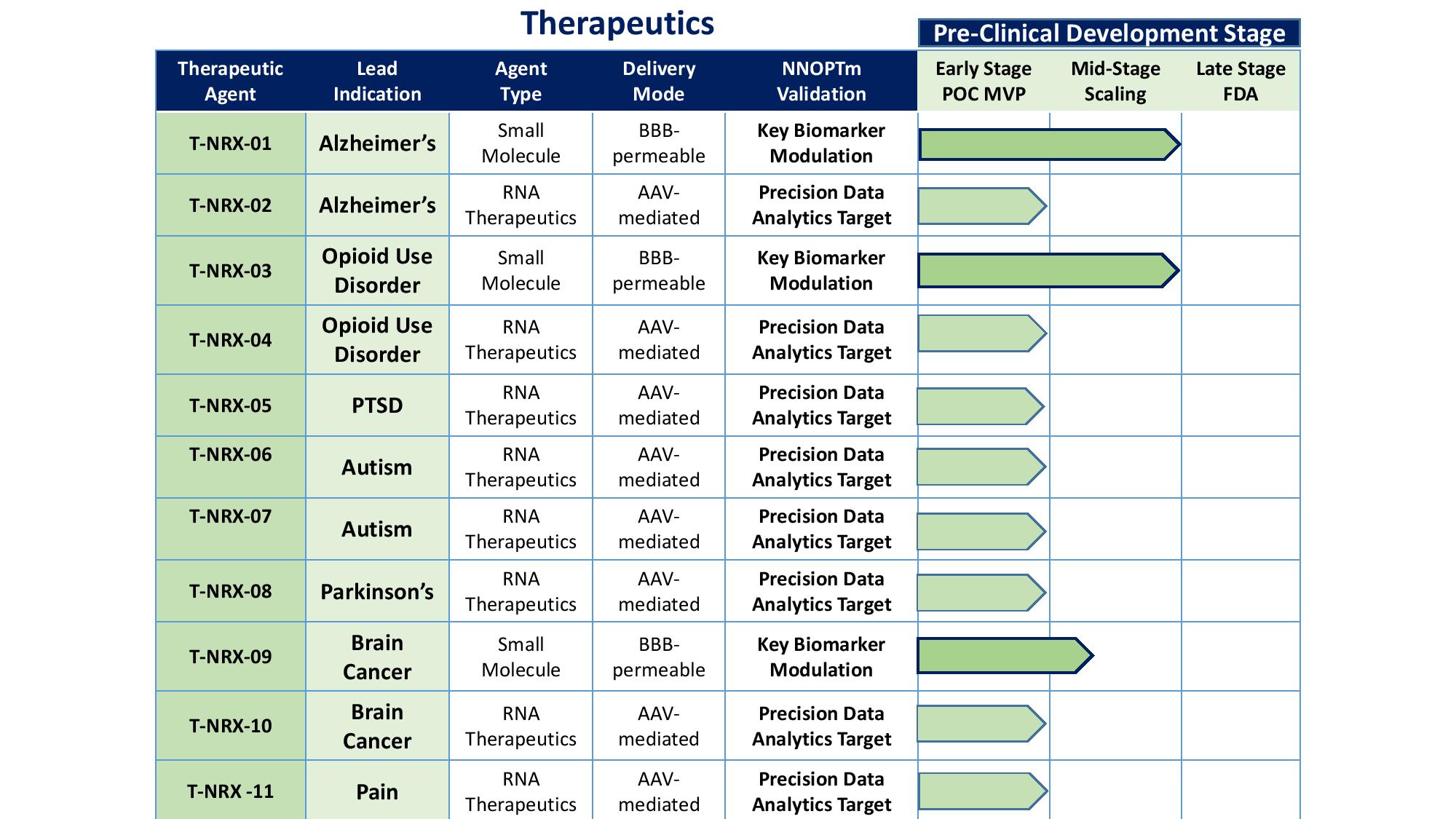
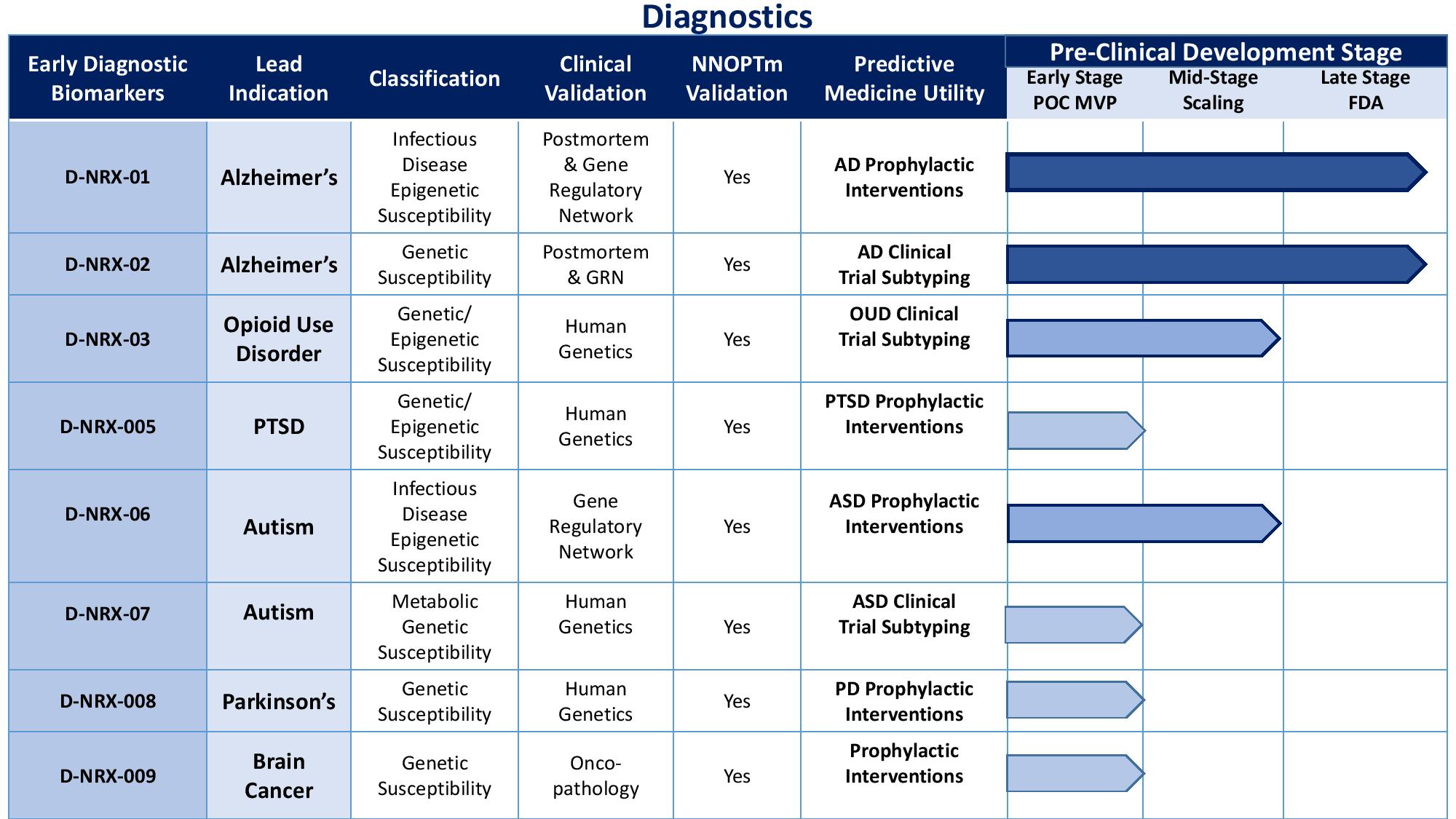
Technology Sublicenses
Neurxstem will sublicense the technology to academic institutions and commercial companies and provide technical support during its initial implementation phase. Please inquire.
Disclaimer
The Neural Organoid Platform™ is for research and development purpose only. Not approved for direct diagnostic or clinical use in humans.
